
Johannes Diderik van der Waals was born on November 23, 1837, into a Dutch middle-class family in Leiden, Netherlands. He was the eldest of ten children born to Elisabeth van den Berg and Jacobus van der Waals who earned his living as a carpenter. In 1853, on completion of his elementary education at Hogere Burgerschool (Higher Civil School) in Leiden, he served as a teacher’s apprentice in an elementary school. Between 1856 and 1861, he took courses to qualify him as an elementary school teacher. To earn his teaching certificate in mathematics and physics at the secondary school level he attended the University of Leiden in his spare time during the three year period spanning 1862 through 1865.
In 1865, Johannes married 18-year-old Anna Magdalena Smit, who became the love of his life. In that year, he was also appointed physics teacher at a secondary school in Deventer. In 1866 he moved to The Hague, first as teacher and later as Director of one of the secondary schools in that town. Meanwhile, he continued his study of physics and on June 14, 1873, was awarded his Ph.D. from the University of Leiden for his dissertation titled, “Over de Continuiteit van den Gas- en vloeistoftoestand” (On the continuity of the gas and liquid state), that laid the foundation for his future work in the field of thermodynamics.
In that thesis, Van der Waals introduced the concepts of molecular volume and molecular attraction and presented his famous Van Der Waals Equation of State for real gases, expressed as:
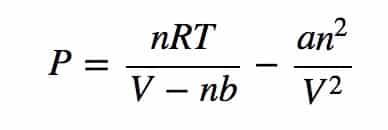
where P is the pressure exerted by a gas, T is its temperature, V is its volume, R is a constant and a, b and n are factors that adjust for the fact that molecules have volume and electromagnetic attraction (now called Van der Waals forces). As a and b approach 0, the equation of state reduces to the ideal gas law.
In September 1877, Van der Waals was appointed the first professor of physics at the Municipal University of Amsterdam. Among his colleagues were physical chemist Jacobus Henricus van’t Hoff and biologist Hugo de Vries. Despite struggling financially, Van der Waals pursued a career in academia and held various teaching positions. In 1880 he published the Law of Corresponding States, which showed that the Van der Waals equation of state can be expressed as a simple function of the critical pressure, critical volume, and critical temperature, thereby generalizing the formula to apply to all substances.
When his wife died of tuberculosis in 1881 at the age of 34, Van der Waals was devastated. He published nothing for another decade. Then in 1890, he published, in the Archives Néerlandaises, a treatise on the Theory of Binary Solutions that connected his Equation of State with the Second Law of Thermodynamics. Van der Waals remained at the University of Amsterdam until his retirement at the age of 70.
In 1910, at the age of 72, Johannes Van der Waals was awarded the Nobel Prize in Physics for his work on the equation of state for gases and liquids. He died on March 8, 1923, at age 85, one year after the death of his daughter, Jacqueline. Van der Waals was succeeded by his son Johannes Diderik van der Waals, Jr., who followed in his father’s footsteps and became a theoretical physicist. The work of Johannes van der Waals advanced significantly our understanding of the behavior of gases and liquids, particularly in the development of the equation of state for gases and the theory of intermolecular forces.
